JICOSH was closed in 2008. For further information, please contact JISHA.
JICOSH Home >
Guidelines >
Report on Results of Chromosome Aberration Test using Cultured Mammalian Cells |
 |
 |
|
Report on Results of Chromosome Aberration Test
using Cultured Mammalian Cells
Attached paper 2 1. General Matters
In
Japanese
|
|
(Attached paper 2)
|
|
Report on Results of Chromosome Aberration Test using Cultured Mammalian
Cells
|
|
1. General Matters
|
Name of the new chemical substance
(IUPAC nomenclature)
|
|
|
Other name
|
|
|
Structural formula or rational
formula (or outline of manufacturing method, in case both are unknown)
|
|
|
Purity of new chemical substance
to be tested
|
wt%
|
Lot No. of new chemical substance
to be tested
|
|
|
Name and content of impurities
|
wt%
|
|
CAS No.
|
|
Vapor pressure
|
|
|
Molecular weight
|
|
Partition coefficient
|
|
|
Melting point
|
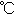
|
Appearance at ordinary temperature
|
|
|
Boiling point
|
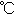
|
|
|
Stability
|
|
|
Solubility, etc. in solvent
|
Solvent
|
Solubility in solvent
|
Stability in solvent
|
Solvent
|
Solubility
|
Stability in solvent
|
|
Water
|
|
|
DMSO
|
|
|
|
Ethanol
|
|
|
Others( )
|
|
|
[Remarks] Enter physicochemical properties as much as possible because they are used as reference.
1. Enter stability to temperature, light, etc. in the
column of "stability".
2. Enter vapor pressure of the test substance in the
column of "vapor pressure".
3. Enter partition coefficient, temperature and name
of the solvent used in the measurement in the column of "partition
coefficient".
4. Enter solubility of the test substance in the solvent
and the stability in the solvent in the column of "solubility, etc.
in solvent".
|
|
|